
Our lab mainly studies the topics in the cell-material interfaces with a focus on mechano-transduction. By combining the theoretical models and biomaterials fabrication, we aim to reveal the mechanism of mechano-transduction in physiological and pathological processes, such as metastasis and immune response, as well as design functional biomaterials. Ultimately, we hope that our studies could provide new clues on disease treatment by regulating the mechano-transduction in biological processes.
1. Cell Mechanosensing
Cells can sense and respond to the mechanical properties, including stiffness and viscoelasticity, of their microenvironment (extracellular matrices). This ability of cell mechanosensing plays an important role in physiological and pathological processes such as tumor formation, wound healing, and tissue fibrosis, which has become a frontier research direction of mechanobiology. Experiments have shown that most of the extracellular matrices (ECMs) exhibit viscoelastic properties, and the matrix viscoelasticity can regulate the stem cell differentiation, cell-cell spreading, and cell migration. Through the combination of theory and experiments, our research group explores the effect of viscoelasticity, viscoplasticity, and other nonlinear mechanical properties of the external matrix on cells. Our research aims to reveal the impact of the previously under-recognized dissipative properties on the dynamic behavior of cells, offering new ideas and theoretical support for the design and optimization of biomaterials.
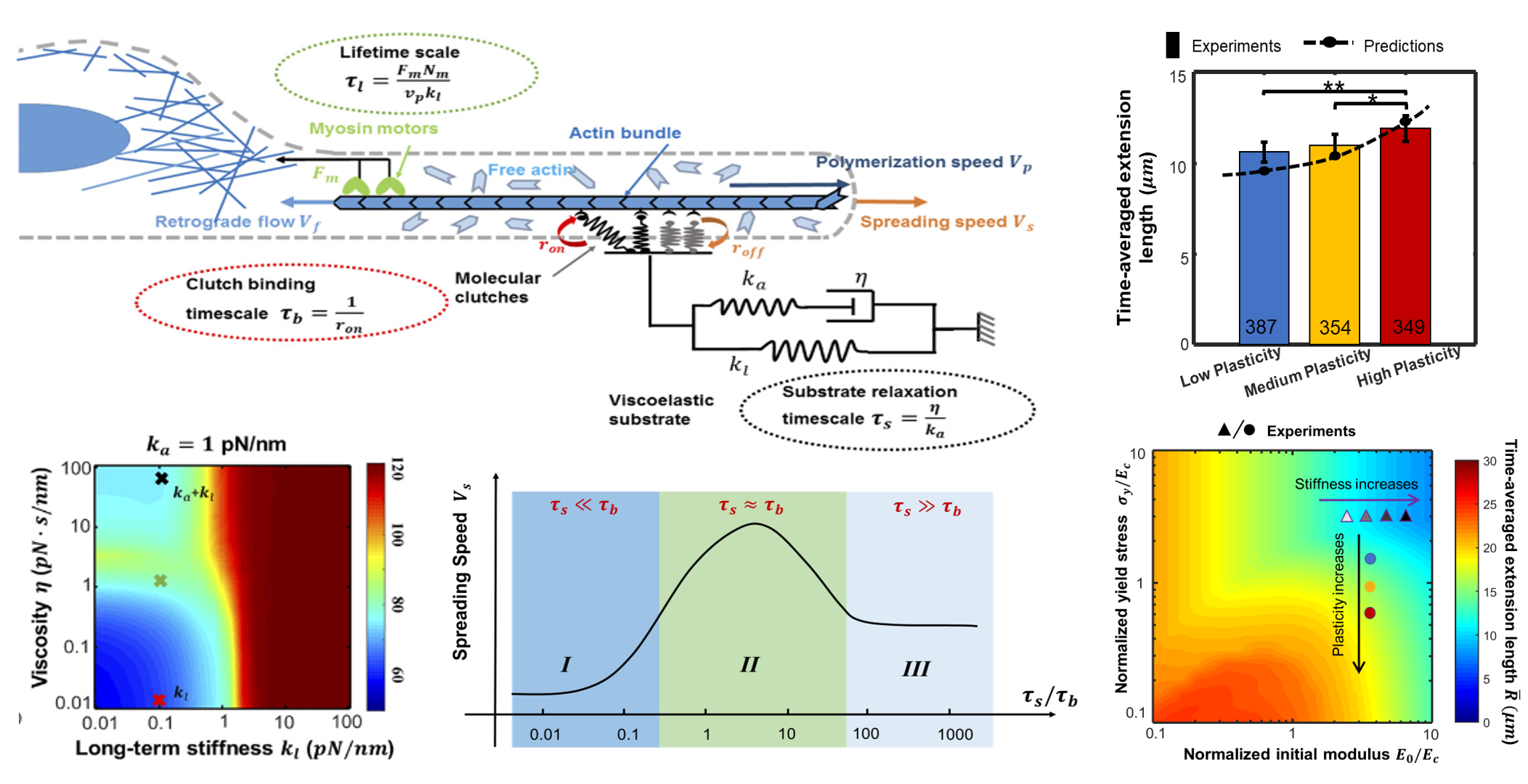
V. Sharma et al., Nat. Commun, 2026; R. Xue et al., ACS Nano, 2024; J. Xing et al., JMPS, 2024; Z. Gong et al., PNAS, 2018; K. Adebowale, Z. Gong et al., Nat. Mater., 2021;
2. Cell Protrusion and Migration
Cell protrusions are highly dynamic, filamentous or sheet-like structures of the cell cytoskeleton, which play a vital role in cell migration and spreading. For example, during tumor metastasis, cancer cells can use invadopodia to mechanically open the matrix cavity and further facilitate their migration and invasion; During immune responses, immune cells can use podosomes to generate invasive force and produce lysozyme to actively modify the surrounding microenvironment, which in turn helps immune cells migrate and locate pathogens. By combining theory and experiments, our lab has revealed the mechanism of cell protrusion dynamics as well as the mechanism of protrusion mechanosensing. Our lab aims to clarify the mechanism of mechanotransduction in the interaction between cell protrusions and the microenvironment and to understand the role of the mechanotransduction mechanism in cancer cell invasion, wound healing, and immune responses.
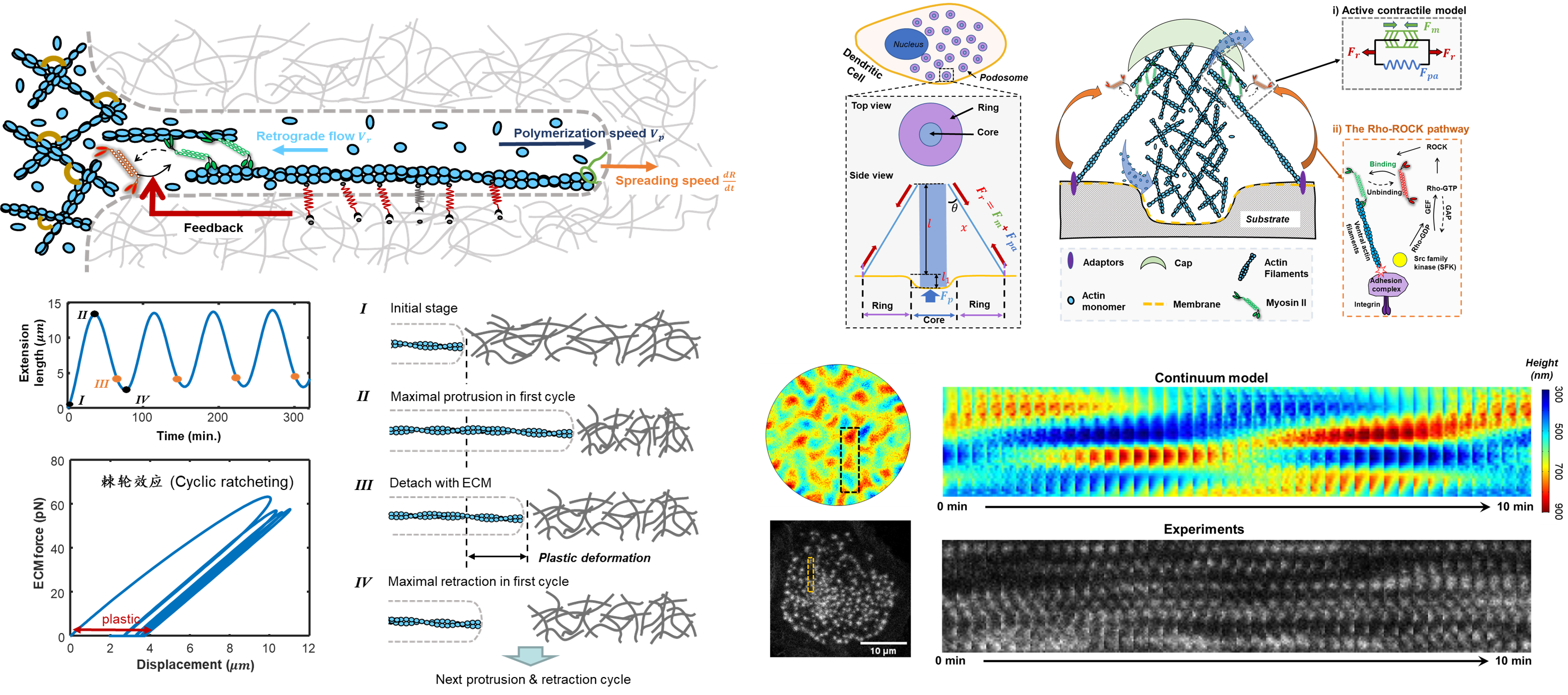
J. Feng et al, PNAS, 2025; J. Zhao et al., Commun. Phys., 2025; Z. Gong, et al., Nat. Commun, 2023; Z. Gong et al., Cell Rep., 2021;
3. Characterization of Mechanical Properties of Cells and Tissues
The mechanical properties of the microenvironment around living cells affect the behavior and pathological changes of cells and tissues. The quantification of the properties of viscoelasticity and nonlinear elasticity in normal and diseased tissues is of great importance. Our research group mainly built an indentation rheological test platform for atomic force microscopy (AFM), using this rheological platform to measure the viscoelasticity of nueron cell cytoskeleton and axons, and quantify the correlation between viscoelasticity and Alzheimer's disease. In addition, our research group used AFM probes to test the nerve injury in the axon provocation and shearing experiments of nerve cells, and combined with the mechanical model to analyze the response of nerve axons after injury. We aim to quantify the dissipative mechanical properties of cells and explore its correlation with brain diseases, such as Alzheimer's disease, which also provides new ideas for future related clinical treatment.
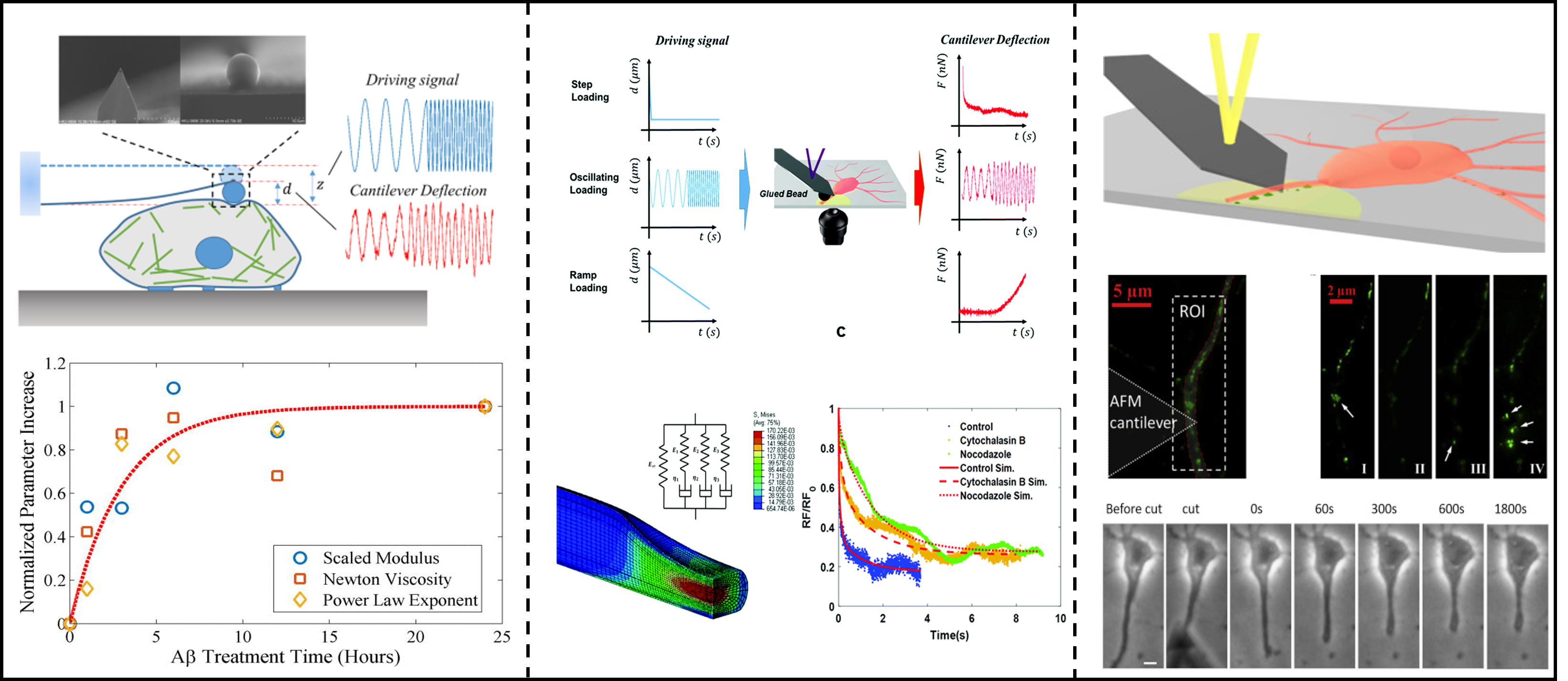
Y. Chen et al, J. Phys. Chem. Lett., 2025; Z. Gong et al., Extreme Mech. Lett., 2020; Z. Gong et al., Soft Matter.,2019 (封面) ; Z. Gong, et al., J. Appl. Phys.,2016;
Selective publications are listed below, and the full publication list can be found on Google Scholar profile
[10] R. Xue#, L. Kang#, Y. Chen, H. Yang, H. Jiang*, Z. Gong*, Force loading on molecular clutches governs the stability of cell lamellipodia, bioRxiv, 10.679903 (2025)
[9] J. Xing, F. Sun, Y. Lin*, Z. Gong*, A chemo-mechanical model for growth and mechanosensing of focal adhesion, Journal of the Mechanics and Physics of Solids, 193, 105863,(2024)(公众号简介)
[8] R. Xue, Y. Chen, Z. Gong*, H. Jiang*, Superposition of Substrate Deformation Fields Induced by Molecular Clutches Explains Cell Spatial Sensing of Ligands, ACS Nano, 18 (32), 21144-21155,(2024)(公众号简介)
[7] Z. Gong, K. van den Dries, R.A. Migueles-Ramírez, P.W. Wiseman, A. Cambi, V.B. Shenoy*. Chemo-mechanical Diffusion Waves Explain Collective Dynamics of Immune Cell Podosomes. Nature Communications, 14, 2902, (2023).
Faculty Opinion: Recommendation of the Article: Facultyopinions.com/article/742656105
[6] K. Adebowale, Z. Gong, J. Hou, K.M. Wisdom, D. Garbett, H. Lee, S. Nam, T. Meyer, D. Odde, V.B. Shenoy, O. Chaudhuri*. Enhanced substrate stress relaxation promotes filopodia-mediated cell migration. Nature Materials, 20, 1290–1299, (2021).
Stanford News: Stanford Study reveals a unique mode of cell migration on soft 'viscoelastic' surfaces
[5] Z. Gong, K.M. Wisdom, E. McEvoy, J. Chang, K. Adebowale, C.C. Price, O. Chaudhuri, V.B. Shenoy*. Recursive Feedback between Matrix Dissipation and Chemo-mechanical Signaling Drives Oscillatory Growth of Cancer Cell Invadopodia. Cell Reports, 35 (4) 190947, (2021).
[4] Z. Gong*, C. Fang, R. You, X. Shao, R. C.C. Chang, Y. Lin*. Forced Peeling and Relaxation of Neurite Governed by Rate-Dependent Adhesion and Cellular Viscoelasticity, Extreme Mechanics Letters, 40, 100902, (2020).
[3] Z. Gong#, C. Fang#, R. You, X. Shao, X. Wei, R.C.C. Chang, and Y. Lin*. Distinct Relaxation Timescales of Neurites Revealed by Indentation under Different Loading Modes. Soft Matter, 15 (2), 166-174, (2019). (Front Cover)
[2] Z. Gong, S.E. Szczesny, S.R. Caliari, E.E. Charrier, O. Chaudhuri, X. Cao, Y. Lin*, R.L. Mauck, P.A. Janmey, J.A. Burdick, and V.B. Shenoy*. Matching Material and Cellular Timescales Maximizes Cell Spreading on Viscoelastic Substrates. Proceedings of the National Academy of Sciences USA, 115 (12), E2686-E2695, (2018).
Penn News: Penn Researchers Show that Cells' Perception of Stiffness is a Matter of Time
HKU News: HKU scientists reveal how material viscosity modulates living cells behavior and functioning
[1] Z. Gong#, R. You#, R.C.-C. Chang, Y. Lin*, Viscoelastic Response of Neural Cells Governed by the Deposition of Amyloid-β Peptides (Aβ), Journal of Applied Physics, 119(21) 214701, (2016).
No content
No content
