guochang
- Supervisor of Doctorate Candidates
- Supervisor of Master's Candidates
- Name (English):Chang Guo
- Name (Pinyin):guochang
- E-Mail:
- Education Level:Postgraduate (Doctoral)
- Business Address:Office 5011, Material Science Building (East Campus), University of Science and Technology of China, Hefei, Anhui, 230026, P. R. China
- Contact Information:Tel:86-(0)551-63601526 E-mail:guochang@ustc.edu.cn
- Degree:Dr
- Alma Mater:University of Science and Technology of China
- Teacher College:Hefei National Research Center for Physical Sciences at the Microscale
 Contact Information
Contact Information
No content
- RESEARCH
Our group specializes in the field of asymmetric electrocatalysis and radical reactions. Our primary research interest lies in advancing novel asymmetric catalysis, with a particular emphasis on the pivotal role that electrochemistry-driven reactive intermediates play in the catalytic process. This approach facilitates the creation of stereogenic centers in enantioenriched products. We are dedicated to discovering and developing innovative methods for asymmetric electrosynthesis, thereby broadening the scope and enhancing the sustainability of synthetic chemistry.
Asymmetric electrosynthesis
Our group is developing general electrocatalytic methods for the diversity-oriented regio- and stereoselective functionalization in organic synthesis.
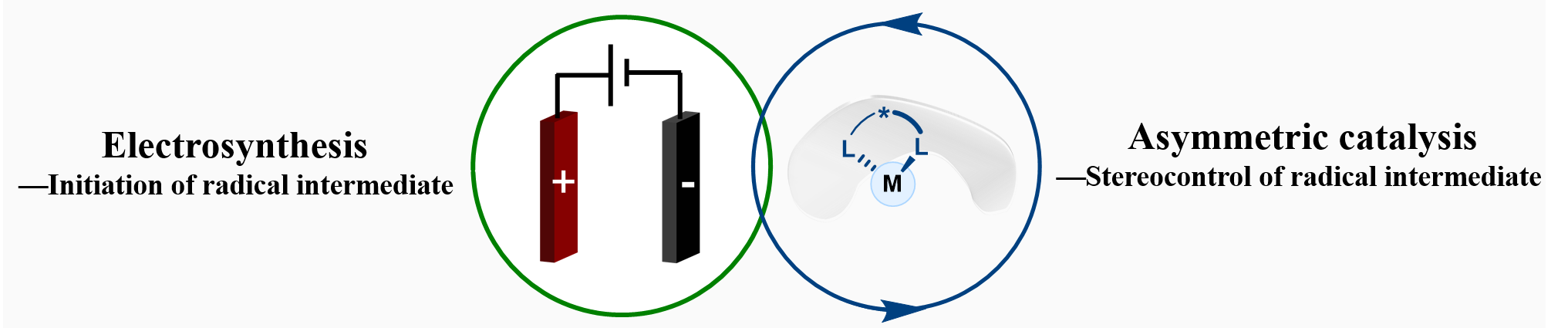
The Chiral Lewis Acid-Coupled Electron Transfer (LCET) process is a catalytic strategy that enables precise enantiocontrol in radical reactions. In this mechanism, a substrate binds to a chiral Lewis acid catalyst, forming an activated complex with a lowered oxidation potential. This complex is then selectively oxidized at the anode to generate a key radical intermediate, which remains templated within the chiral pocket of the catalyst. Finally, this chaperoned radical undergoes stereocontrolled bond formation with a coupling partner. This strategy effectively overcomes the inherent challenge of controlling stereochemistry in reactions involving highly reactive radical species.

Asymmetric propargylation
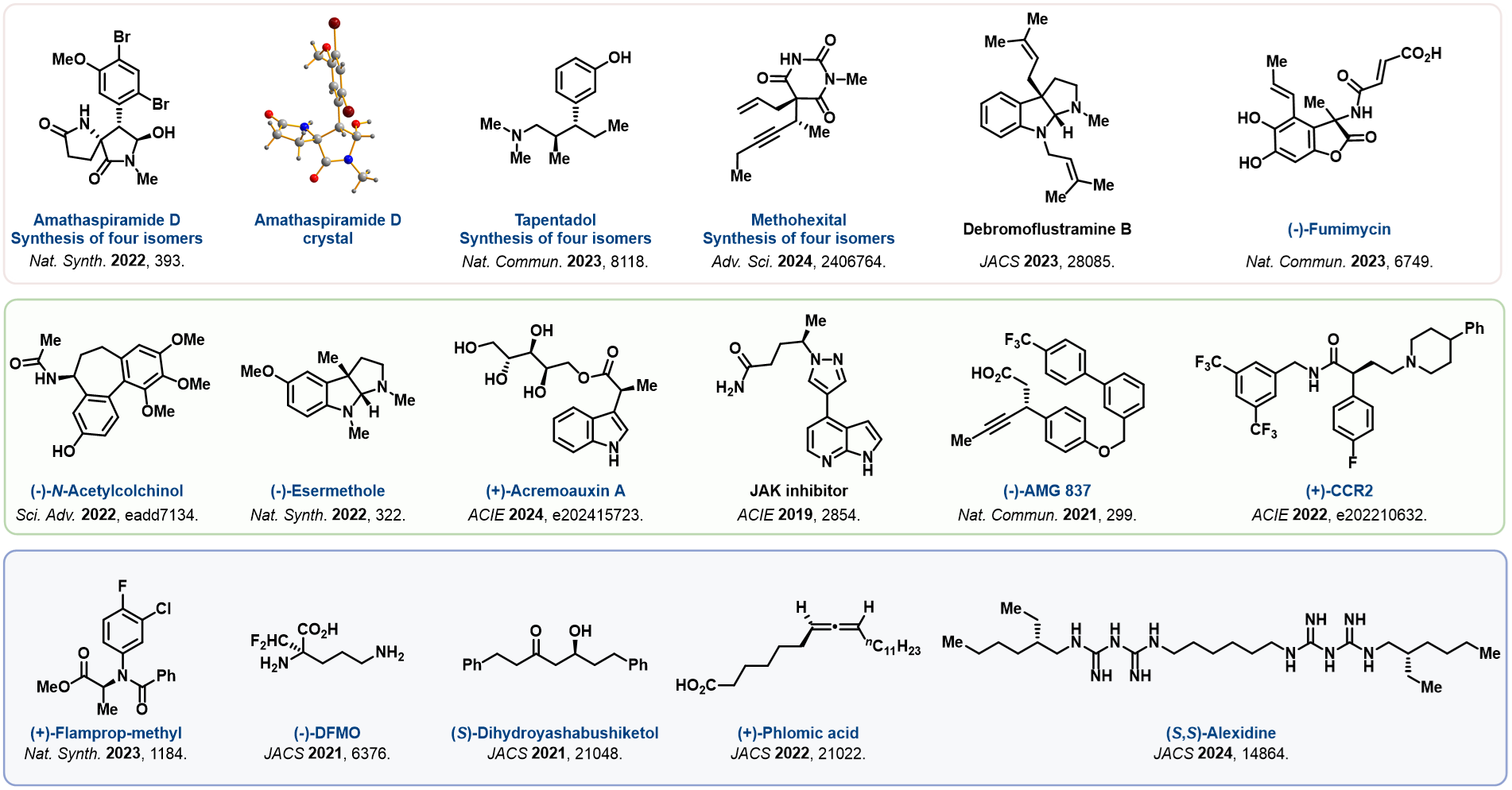
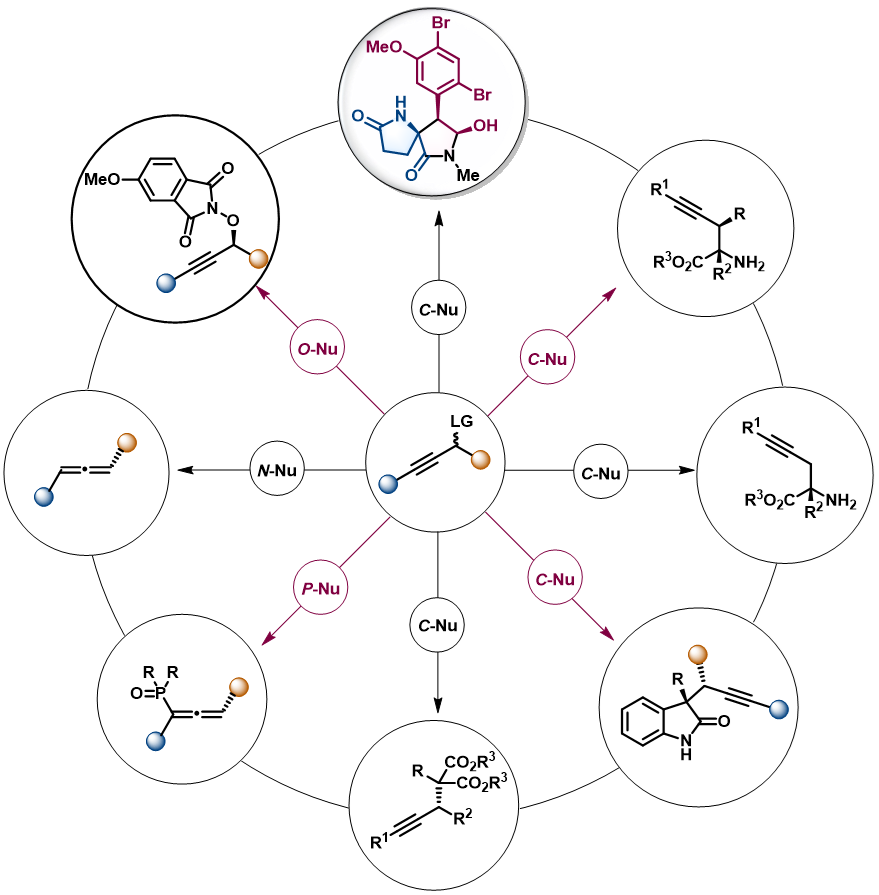
The group is also involved in asymmetric propargylation to achieve divergent synthesis of natrual products.
Total synthesis
One of the main areas of our research is the total synthesis of structrually complex, bioactive naturual products, and pharmaceuticals.