
Scientific Research
Research Field
Saturated carbon-carbon bonds are ubiquitous in organic molecules, supporting organic molecules to present a three-dimensional structure. The development of stereoselective construction strategies for saturated carbon-carbon bonds has important implications for complex molecular synthesis and retrosynthetic analysis. Highly enantioselective saturated carbon coupling under mild conditions is a straightforward but challenging method for constructing saturated carbon-carbon bonds. The difficulties include: (1) the access to alkyl coupling reagents is limited, highly dependent on highly active metal reagents, lack of commercial reagent sources, and limited functional group compatibility; (2) it is difficult to control the chirality of saturated carbon centers, and it is difficult to obtain high enantioselective products. Dr. Lu Xi is committed to developing a new reaction with high selectivity and simple steps in line with the concept of green chemistry - alkene hydroalkylation, which partially solves the key problems in the field of saturated carbon center coupling.
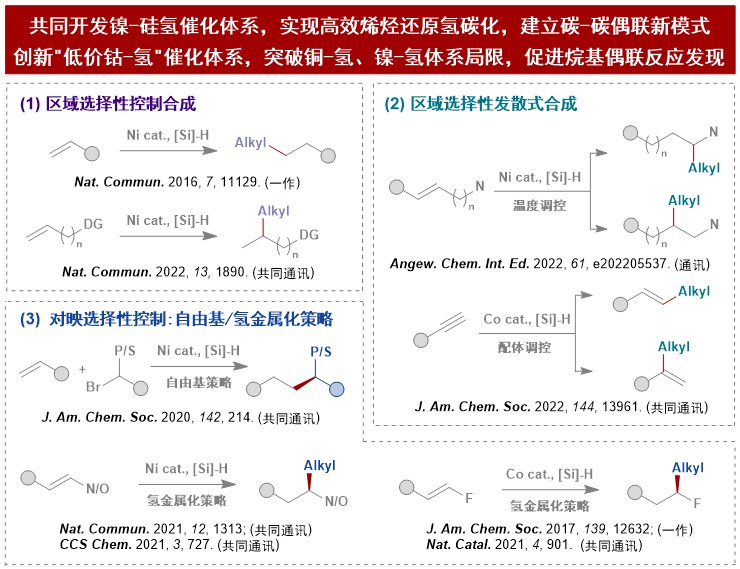
Dr. Xi Lu carried out metal hydride-catalyzed alkene hydroalkylation and its mechanism research, precisely controlled the regioselectivity and enantioselectivity, clarified the reaction mechanism and answered the common problems of selectivity. Specifically include: (1) create a nickel-silane catalytic system to achieve efficient olefin reductive hydrocarbonation, and establish a new carbon-carbon coupling model; (2) systematically develop a nickel-hydride catalytic system to achieve regio- and enantioselective alkene hydroalkylation; (3) innovative cobalt-hydride catalytic system, the coupling activity and selectivity are significantly different from copper-hydride and nickel-hydride systems, breaking through the limitations of traditional auxiliary groups for asymmetric cross-coupling, facilitating discovery of saturated carbon coupling reactions.
Paper Publications
+more
-
Shi-Jiang He, Jia-Wang Wang, Yan Li,Zhe-Yuan Xu, Xiao-Xu Wang.Xi Lu, Yao Fu .Nickel-Catalyzed Enantioconvergent Reductive Hydroalkylation of Olefins with α‑Heteroatom Phosphorus or Sulfur Alkyl Electrophiles.J. Am. Chem. Soc,2020,142214-221.
-
Xi Lu,Yan Wang, Ben Zhang, Jing-Jing Pi, Xiao-Xu Wang, Bin Xiao.Tian-Jun Gong, Yao Fu.Nickel-Catalyzed Defluorinative Reductive Cross-Coupling of gem-Difluoroalkenes with Unactivated Secondary and Tertiary Alkyl Halides.J. Am. Chem. Soc,2017,13912632-12637.
-
Xiao-Xu Wang, Yuan-Tai Xu,Zhi-Lin Zhang.Xi Lu, Yao Fu .NiH-catalysed proximal-selective hydroalkylation of unactivated alkenes and the ligand effects on regioselectivity.Nature Commun.,2022,131890.
-
Jia-Wang Wang, Yan Li,Wan Nie, Zhe Chang, Zi-An Yu, Yi-Fan Zhao.Xi Lu, Yao Fu .Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines.Nature Commun.,2021,121313.
-
Xi Lu, Bin Xiao,Zhenqi Zhang, Tianjun Gong, Wei Su, Jun Yi.Yao Fu, Lei Liu.Practical carbon–carbon bond formation from olefins through nickel-catalyzed reductive olefin hydrocarbonation.Nature Commun.,2016,711129.
-
Xiao-Xu Wang, Lu Yu,Zhi-Lin Zhang, De-Guang Liu, Changlin Tian.Xi Lu, Yao Fu .NiH-Catalyzed Reductive Hydrocarbonation of Enol Esters and Ethers.CCS Chem.,2022,4605-615.
Patents
Published Books
Research Projects
|
-
Xi Lu
 ZipCode:52c9ab2533ce514a1a885e74a1342fc6b73de36e785baa3e07cdd5a7b1234d742197243126974fa2d7f664ed7d2e5a67cbd4c522a53cdf7d80ceb39928c7039b528bb15c9ba80a39ab0e149f9b2e9c09f59aa8081c897dd70ff6d191397c82f7cd3a3befe211634c0cd859c7c66d7460ad307de7bcbf3431ed677f37d4784f77  PostalAddress:c2631deed75eb79b1f0e5a0aa813a3ef611fd7ee388a3f03acba8c246790b569d6249eb9b2c05f89b935bc8c2aeac3775ba6b7549e41501ae50787e9bfafe4036195c74bcf0e5bf7b628eca59771f576b8913b336025a9b274bfb55b6ace4c99ee9ffb8dbd77377345b7b07bc7da17e9452a47be8e5dafcdee2ecfdf4b3f6b3e  Email:c16f92875588528e58b818ad9d447064ee290a1383dd66f456b9eea5ab64427d24636ad1e749a0de0b90e3a48735999de07e75252050823f3cce4aa10cf6702034efa6366ea182ab03e85effce1ab3f271f0ebc50f1280f618a69151d8171a8776b2b0cc5452a7ca5b80bbe2897549cedf61876502c1429a5861aa2e49450fec
|



