公泽
开通时间:..
最后更新时间:..
公泽教授课题组围绕生物力学、力学生物学领域中的前沿问题展开多学科交叉研究,旨在通过建立多尺度力学生物学模型、设计生物软材料、生化实验等手段,探究组织形态的发育、体内再生过程和癌症扩散等生理与病理过程中的力学传导,阐明其中的力学生物学的机理,并进一步结合生物材料制备、3D打印等技术设计和研发更为接近体内细胞生存环境的功能性生物材料,探索调控生命体的力学传导来治疗疾病的新思路。
1.细胞的力学感知
细胞能够感知和响应细胞周围微环境(外基质)的硬度、粘弹性等力学属性,这一细胞力学感应现象在肿瘤形成、伤口愈合和组织纤维化等生理和病理过程中起到重要作用,该方向也是生物力学和力学生物学前沿研究领域。实验表明绝大部分外基质呈现粘弹性等耗散力学属性, 并且基质的粘弹性等力学特性可以调控干细胞的分化、细胞铺展以及迁移。我们课题组主要通过构建细胞力学感应的力学生物学模型,结合细胞生化实验和制备可调控粘弹性水凝胶,探究外基质的粘弹性、塑性等非线性力学特性对细胞的影响。我们研究旨在揭示此前未被充分认识的外基质耗散力学特性对细胞动态行为影响,为生物软材料设计和优化提供了新思路和理论支持。
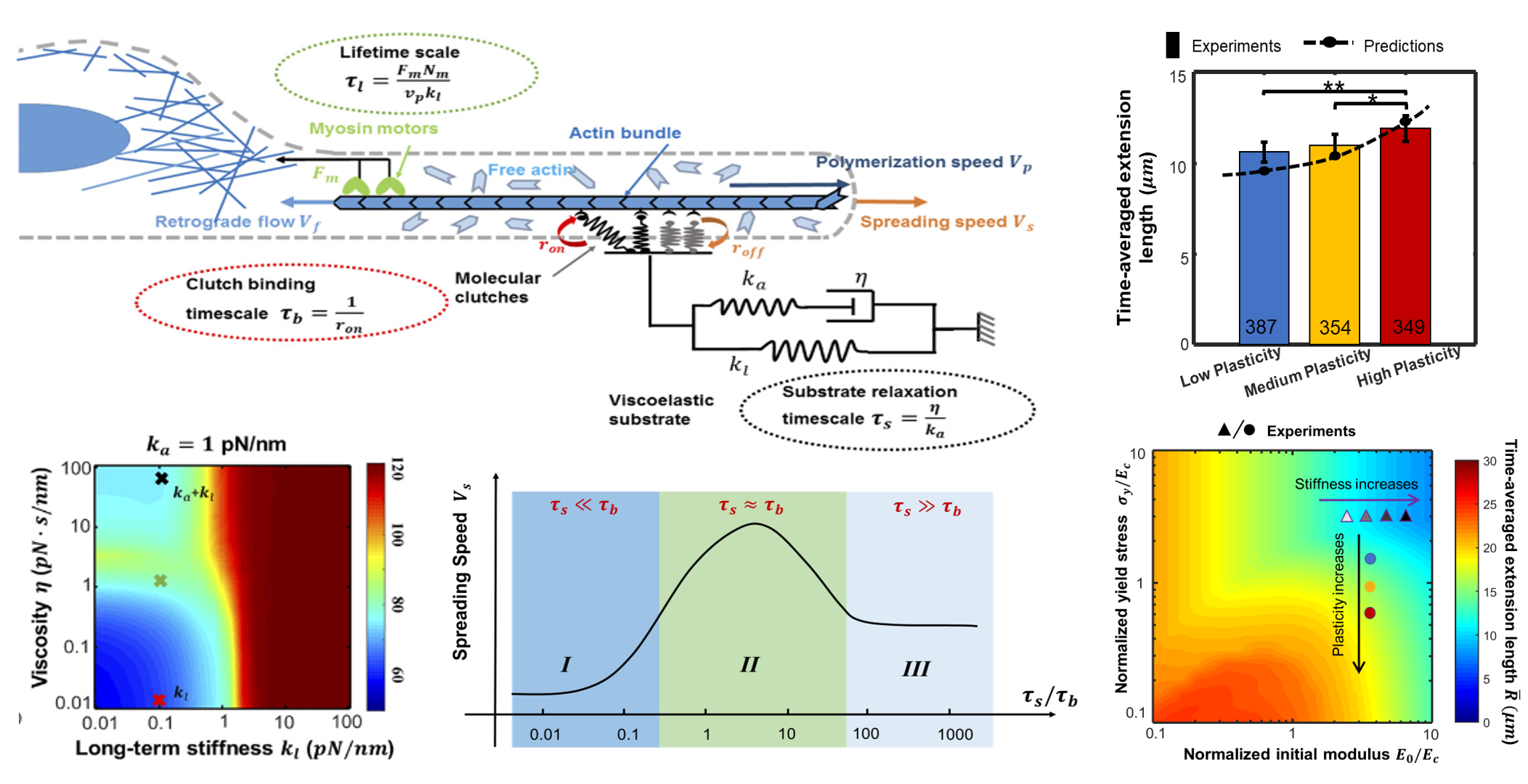
V. Sharma et al., Nat. Commun, 2026; R. Xue et al., ACS Nano, 2024; J. Xing et al., JMPS, 2024; Z. Gong et al., PNAS, 2018; K. Adebowale, Z. Gong et al., Nat. Mater., 2021;
2.细胞迁移和伪足动态
细胞的伪足是细胞生长出来的丝状或者片状的类似足体的结构,其在细胞迁移和铺展等过程起到至关重要的作用。比如,肿瘤转移的第一步是癌细胞利用侵入式伪足(invadopodia)打开基质空腔以方便自身的迁移和入侵,而免疫细胞可以利用足体(podosome)来产生侵入力并生产分解酶改造周围的微环境,进而帮助免疫细胞的迁移和寻找病原体。我们课题组主要通过理论结合实验的方式,揭示了细胞中的伪足动态生长形成机理,以及其对外基质的力学特性的力学响应机理。该研究方向旨在阐明细胞伪足与微环境相互作用中的力学传导的机理,了解力学传导机理在癌细胞侵袭、伤病愈合、以及免疫识别中作用。
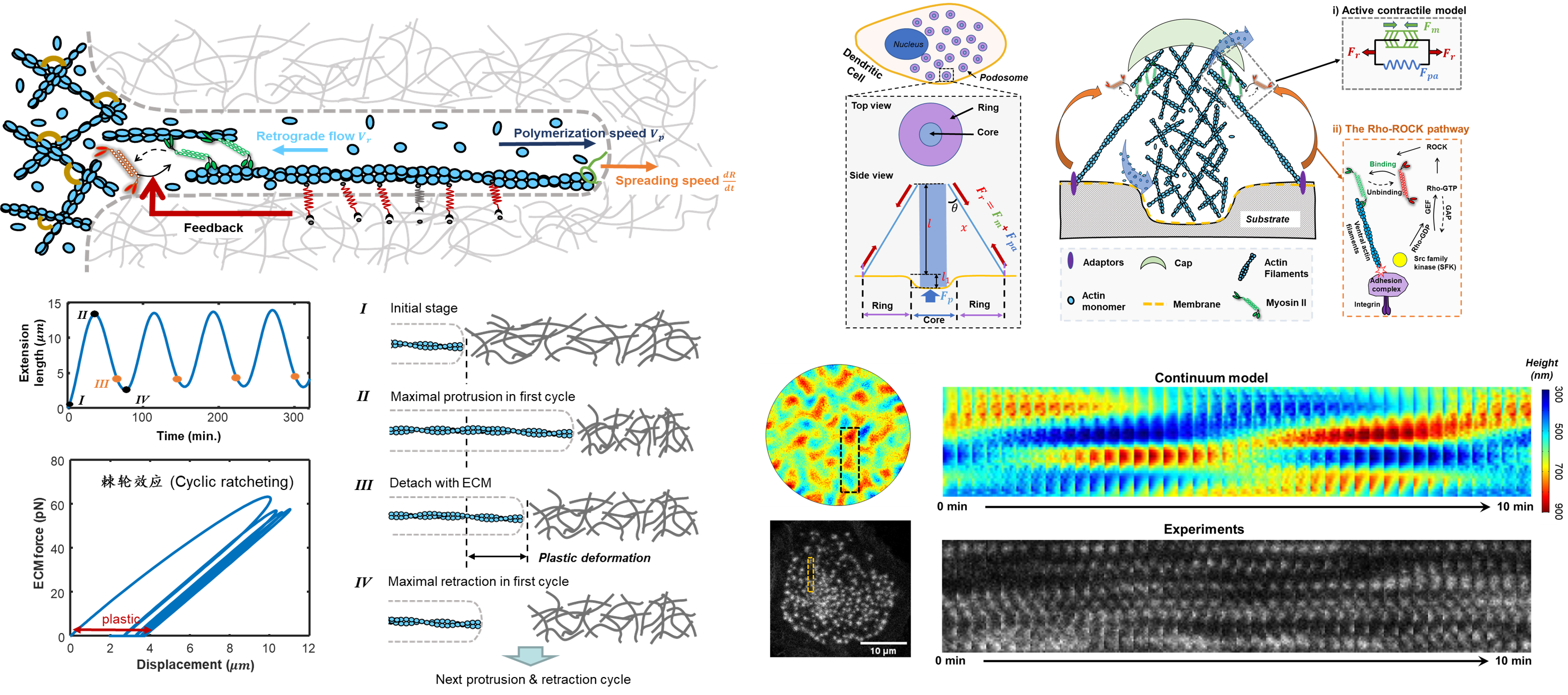
J. Feng et al, PNAS, 2025; J. Zhao et al., Commun. Phys., 2025; Z. Gong, et al., Nat. Commun, 2023; Z. Gong et al., Cell Rep., 2021;
3.细胞和组织非线性力学特性的表征
细胞周围的微环境的力学特性对细胞组织的行为和病变等有着重要的影响,正常和患病组织中的粘弹性、非线性弹性的性质未得到充分量化表征。我们课题组主要搭建了原子力显微镜(AFM)的压痕流变测试平台,利用该流变学平台测量神经细胞胞体骨架和轴突的粘弹性,量化粘弹性与阿兹海默症的相关性。此外,我们课题组利用AFM探针对神经细胞的轴突挑起和剪切实验进行神经损伤测试,结合力学模型分析神经轴突损伤后响应。该方向旨在量化细胞耗散性力学属性,并探究其与阿兹海默症等脑类疾病的相关性,为阐明脑类疾病致病机理提供了重要力学生物学依据,也对未来相关临床治疗提供新的思路
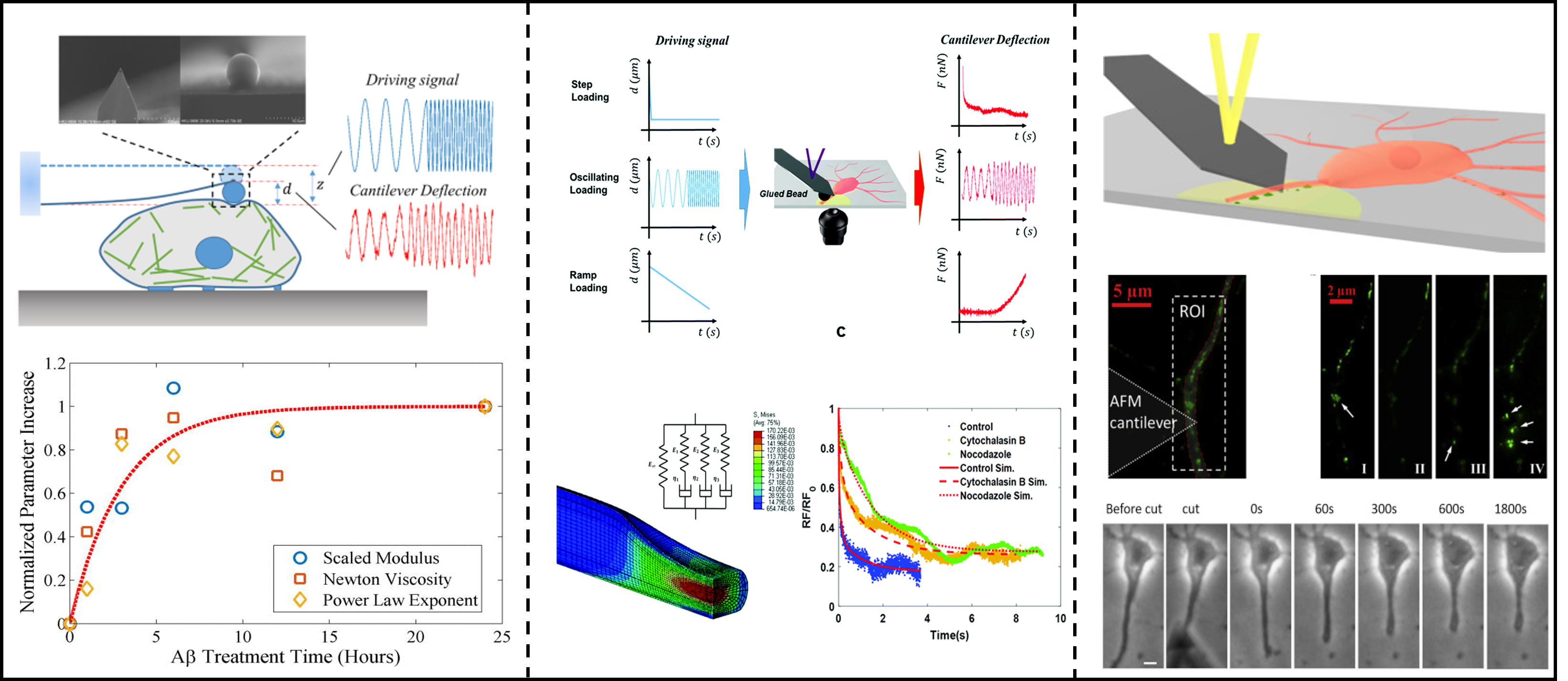
Y. Chen et al, J. Phys. Chem. Lett., 2025; Z. Gong et al., Extreme Mech. Lett., 2020; Z. Gong et al., Soft Matter.,2019 (封面) ; Z. Gong, et al., J. Appl. Phys.,2016;
Dr. Gong's full publication list can also be found on his Google Scholar profile
近期发表论文(2023-2026)
[24] V. Sharma#, K. Adebowale#, Z. Gong#, Ovijit Chaudhuri*, Vivek Shenoy*, Glassy Adhesion Dynamics Govern Transitions Between Sub-Diffusive and Super-Diffusive Cell Migration on Viscoelastic Substrates, Nature Communications, In Press
[23] R. Xue#, L. Kang#, Y. Chen, H. Yang, H. Jiang*, Z. Gong*, Force loading on molecular clutches governs the stability of cell lamellipodia, bioRxiv, 10.679903 (2025)
[22] P. Zhao#, Z. Zhang#, R. Xue#, Y. Zheng, Y. Gao, J. Cao, M. Jiang, Y. Jiang, Y. Zha, L. Gao, Z. Gong*, J. Du*, Y. Cao*, Contact stiffness governs cell mechanosensing through molecular clutches, Communications Biology, 8 (1), 1670,(2025)
[21] J. Feng#, K. Feng#, Z. Xiong, M. Yu, Y. Hu, W. Wang, R. Xue, Z. Gong*, Z. Liu*, W. Chen*, Capped high-force integrin bond lifetimes and spacing-tuned binding frequency drive rapid fibroblast migration, Proceedings of the National Academy of Sciences USA, 122 (47), e2505941122,(2025)(公众号简介)
[18] G. Wang, M. Yang, Z. Gong, C. Li*, Visible Light‐Driven Spatiotemporal‐Resolved Proton Management by Harnessing Merocyanine‐Integrated Hydrogels as Regenerative Photoacid Matrices, Advanced Materials, 37 (40), e08265(2025)
[20] Y. Chen, R. Xue, Z. Gong*, H. Jiang*, Successive Spin Coating Induces an Order-Disorder Transition in a Block Copolymer Micellar Nanoarray, The Journal of Physical Chemistry Letters, 16 (36), 9463-9469,(2025)(公众号简介)
[19] J. Zhao, H. Zhang, Y. Yang, R. Xue, Z. Gong*, H. Jiang*, Ca2+ transmembrane transport enhances oscillatory growth of cancer cell invadopodia, Communications Physics, 8 (1), 346, (2025)(公众号简介)
[17] J. Xing, F. Sun, Y. Lin*, Z. Gong*, A chemo-mechanical model for growth and mechanosensing of focal adhesion, Journal of the Mechanics and Physics of Solids, 193, 105863,(2024)(公众号简介)
[16] R. Xue, Y. Chen, Z. Gong*, H. Jiang*, Superposition of Substrate Deformation Fields Induced by Molecular Clutches Explains Cell Spatial Sensing of Ligands, ACS Nano, 18 (32), 21144-21155,(2024)(公众号简介)
[15] Z. Gong, K. van den Dries, R.A. Migueles-Ramírez, P.W. Wiseman, A. Cambi, V.B. Shenoy*. Chemo-mechanical Diffusion Waves Explain Collective Dynamics of Immune Cell Podosomes. Nature Communications, 14, 2902, (2023).
Faculty Opinion: Recommendation of the Article: Facultyopinions.com/article/742656105
[14] Q. Zhang, D. Lee, L. Zheng, X. Ma, S. I. Meyer, L. He, H. Ye, Z. Gong, B. Zhen, K. Lai, A. C. Johnson, Gigahertz topological valley Hall effect in nanoelectromechanical phononic crystals, Nature Electronics, 5 (3), 157-163, (2022).
早期研究工作 (2022年5月前)
[13] K. Adebowale, Z. Gong, J. Hou, K.M. Wisdom, D. Garbett, H. Lee, S. Nam, T. Meyer, D. Odde, V.B. Shenoy, O. Chaudhuri*. Enhanced substrate stress relaxation promotes filopodia-mediated cell migration. Nature Materials, 20, 1290–1299, (2021).
Stanford News: Stanford Study reveals a unique mode of cell migration on soft 'viscoelastic' surfaces
[12] Z. Gong, K.M. Wisdom, E. McEvoy, J. Chang, K. Adebowale, C.C. Price, O. Chaudhuri, V.B. Shenoy*. Recursive Feedback between Matrix Dissipation and Chemo-mechanical Signaling Drives Oscillatory Growth of Cancer Cell Invadopodia. Cell Reports, 35 (4) 190947, (2021).
[11] F. Alisafaei#, Z. Gong#, V. Johnson, J. Dolle, D. Smith, V. B. Shenoy*. Mechanisms of Local Stress Amplification in Axons Near the Gray-White Matter Interface. Biophysical Journal, 119 (7), 1290-1300, (2020).
New and Notable: Multiscale mechanobiology of brain injury: axonal strain redistribution
[10] Z. Gong*, C. Fang, R. You, X. Shao, R. C.C. Chang, Y. Lin*. Forced Peeling and Relaxation of Neurite Governed by Rate-Dependent Adhesion and Cellular Viscoelasticity, Extreme Mechanics Letters, 40, 100902, (2020).
[9] K. Mandal*, Z. Gong, A. Rylander, V.B. Shenoy, P.A. Janmey*. Opposite Responses of Normal Hepatocytes and Hepatocellular Carcinoma Cells to Substrate Viscoelasticity, Biomaterials Science, 8, 1316-1328, (2020).
[8] H. Liu#, C. Fang#, Z Gong, R.C.C. Chang, J. Qian, H. Gao*, Y. Lin*, Fundamental Characteristics of Neuron Adhesion Revealed by Forced Peeling and Time-Dependent Healing. Biophysical Journal, 118 (8), 1811-1819, (2020).
[7] Z. Gong#, C. Fang#, R. You, X. Shao, X. Wei, R.C.C. Chang and Y. Lin*. Distinct Relaxation Timescales of Neurites Revealed by Indentation under Different Loading Modes. Soft Matter, 15 (2), 166-174, (2019). (Front Cover)
[6] P. Pakshir, M. Alizadehgiashi, B. Wong, N. M. Coelho, X. Chen, Z. Gong, V. B. Shenoy, C. McCulloch and B. Hinz*. Dynamic Fibroblast Contractions Attract Remote Macrophages in Fibrillar Collagen Matrix. Nature Communications, 10 (1), 1850, (2019).
[5] X. Shao, R. You, T. H. Hui, C. Fang, Z. Gong, Z. Yan, R.C.C. Chang, V. B. Shenoy*, Y. Lin*. Tension and Adhesion Regulated Retraction of Injured Axons. Biophysical Journal, 117, 193-202, (2019)
[4] Z. Gong, S.E. Szczesny, S.R. Caliari, E.E. Charrier, O. Chaudhuri, X. Cao, Y. Lin*, R.L. Mauck, P.A. Janmey, J.A. Burdick and V.B. Shenoy*. Matching Material and Cellular Timescales Maximizes Cell Spreading on Viscoelastic Substrates. Proceedings of the National Academy of Sciences USA, 115 (12), E2686-E2695, (2018).
Penn News: Penn Researchers Show that Cells' Perception of Stiffness is a Matter of Time
HKU News: HKU scientists reveal how material viscosity modulates living cells behavior and functioning
[3] L. Yang*, Z. Gong, Y. Lin*, V. Chinthapenta, Q. Li, T. J. Webster, and B.W. Sheldon*. Disordered Topography Mediates Filopodial Extension and Morphology of Cells on Stiff Materials. Advanced Functional Materials, 27, 1702689, (2017).
[2] B. Chong, Z. Gong, Y. Lin*, Modeling the Adhesive Contact between Cells and a Wavy Extracellular Matrix Mediated by Receptor-Ligand Interactions, Journal of Applied Mechanics, Transactions ASME, 84 (1), (2017).
[1] Z. Gong#, R. You#, R.C.-C. Chang, Y. Lin*, Viscoelastic Response of Neural Cells Governed by the Deposition of Amyloid-β Peptides (Aβ), Journal of Applied Physics, 119(21) 214701, (2016).
国家自然科学基金—面上项目,免疫细胞足体感应外基质力学微环境的机理研究,2025.01-2028, 主持
国家自然科学基金—青年科学基金项目,三维微环境中细胞对外基质粘弹性力学感应的机理研究,2023.01-2025.12, 主持
中国科学技术大学青年创新重点基金,三维外基质粘弹性调控癌细胞行为的机理探究,2023.01-2024.12,主持
国家重点研发计划—青年科学家项目, 血管化类脑器官多工艺融合生物3D打印研究,2025.01-2029.12,项目骨干
中国科学院B类先导—离体肝脏维持评估与修复再生关键技术专项, 离体肝脏修复再生体系研究,2025.01-2027.12, 项目骨干