特任教授
Supervisor of Doctorate Candidates
Business Address:Room 319, No.1 Mechanics building,University of Science and Technology of China, Hefei, Anhui, China
Contact Information:gongze@ustc.edu.cn
Degree:博士
Alma Mater:University of Hong Kong
The Last Update Time: ..
Our lab mainly studies the topics in the cell-material interfaces with a focus on mechano-transduction. By combining the theoretical models and biomaterials fabrication, we aim to reveal the mechanism of mechano-transduction in physiological and pathological processes, such as metastasis and immune response, as well as design functional biomaterials. Ultimately, we hope that our studies could provide new clues on disease treatment by regulating the mechano-transduction in biological process.
1. Cell Mechanosensing
Cells can sense and respond to mechanical properties, including stiffness and viscoelasticity, of their microenvironment (extracellular matrices). This ability of cell mechanosensing plays an important role in physiological and pathological processes such as tumor formation, wound healing and tissue fibrosis, which has become the frontier research direction of mechanobiology. Experiments have shown that most of the extracellular matrices (ECMs) exhibit viscoelastic properties, and the matrix viscoelasticity can regulate the stem cell differentiation, cell cell spreading, and cell migration. Through the combination of theory and experiments, our research group explores the effect of viscoelasticity, viscoplasticity, and other nonlinear mechanical properties of the external matrix on cells. Our research aims to reveal the impact of the previously under-recognized dissipative properties on the dynamic behavior of cells, offering new ideas and theoretical support for the design and optimization of bio-materials.

Z. Gong, et al., PNAS, 2018; Z. Gong, et al, Cell Rep., 2021; K. Adebowale, Z. Gong, et al., Nat. Mater., 2021; K Mandal, Z Gong, et al., Biomater. Sci., 2020;
2. Cell Protrusion and Migration
Cell protrusions are highly dynamic, filamentous or sheet-like structures of cell cytoskeleton, which play a vital role in cell migration and spreading. For example, during tumor metastasis, cancer cells can use invadopodia to mechanically open the matrix cavity and further facilitate their migration and invasion; During immune responses, immune cells can use podosomes to generate invasive force and produce lysozyme to actively modify the surrounding microenvironment, which in turn helps immune cells migrate and locate pathogens. By combining theory and experiments, our lab has revealed the mechanism of cell protrusion dynamcis as well as the mechanism of protrusion mechanosensing the mechanical properties of the extracellular matrices. Our lab aims to clarify the mechanism of mechanotransduction in the interaction between cell protrusions and microenvironment and to understand the role of mechanotransduction mechanism in cancer cell invasion, wound healing, and immune responses.

Z. Gong, et al., Nat. Commun, 2023;Z. Gong, et al., Cell Rep., 2021; L Yang, Z Gong, et al., Adv. Func. Mater., 2017;P Pakshir, et al., Nat. Commun, 2019;
3. Characterization of Mechanical Properties of Cells and Tissues
The mechanical properties of the microenvironment around living cells affect the behavior and pathological changes of cells and tissues. The quantification of the properties of viscoelasticity and nonlinear elasticity in normal and diseased tissues is of great importance. Our research group mainly built an indentation rheological test platform for atomic force microscopy (AFM), using this rheological platform to measure the viscoelasticity of nueron cell cytoskeleton and axons, and quantify the correlation between viscoelasticity and Alzheimer's disease. In addition, our research group used AFM probes to test the nerve injury in the axon provocation and shearing experiments of nerve cells, and combined with the mechanical model to analyze the response of nerve axons after injury. We aim to quantify the dissipative mechanical properties of cells and explore its correlation with brain diseases, such as Alzheimer's disease, which also provides new ideas for future related clinical treatment.
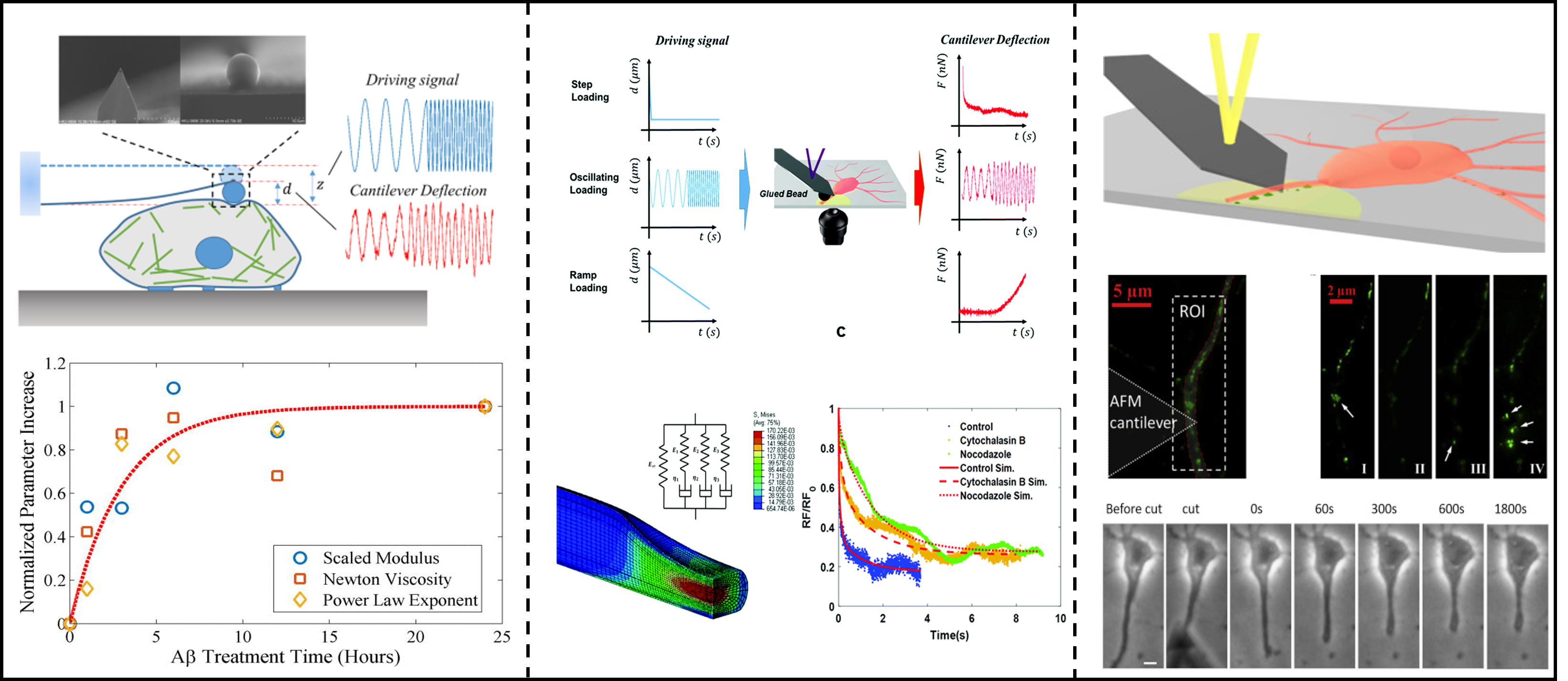
Z. Gong, et al., Soft Matter.,2019 ; Z. Gong*, et al., Extreme Mech. Lett., 2020; Z. Gong, et al., J. Appl. Phys.,2016; H. Liu, C. Fang, Z. Gong, et al., Biophys. J., 2020;